|
Lloyd L. Tran Lloyd L. Tran is a scientist, with more than 25 year experience in drug discovery and business development in pharmaceutical industry. He serves as the Chairman and Chief Scientific Officer of Biomed Industries, Inc. , located in San Jose, California, USA. Biomed Industries, Inc. is the parent company of the following subsidiaries: Biomed Pharmaceutical, Inc., NeuroActiva, Inc., Biomed Green, LLC., Biomed AI, LLC., and MedAware Systems, Inc. In his early career, he was employed as a scientist working on drug discovery at some major pharmaceutical companies including G.D. Searle, Monsanto and Pfizer. Lloyd was the drug developer for Artemether for the treatment of malaria in early 1990’s in collaboration with a Chinese research team. He coordinated the clinical trials of Artemether gel capsule with the support from World Health Organization (WHO) and Walter Reed Army Institute of Research in Washington DC, that led to its approval in over 80 countries worldwide, including the European Medicines Agency (EMA) and the U.S. Food and Drug Administration. The developer of NA-831 for the treatment of Alzheimer's Disease After the success of Artemether, Lloyd began doing research on neurodegenerative diseases when his father developed symptoms of Alzheimer’s disease (AD). There are more than 6.5 million people in the US and 50 million people worldwide have this dreadful disease. Over the past 30 years, it was believed that Alzheimer’s disease is caused by the building up of a certain protein, called amyloid beta, that forms an amyloid plaque in the brain. This amyloid hypothesis has been the guiding pathway for almost all drug development in Alzheimer research. All drugs based on amyloid hypothesis failed. Aducanumab and Lecanemab were approved by the FDA in June 2021 and 2023 respectively. The US Centers for Medicare and Medicaid Services (CMS, or Medicare) ruled that due to its serious adverse effect and lack of benefits to patients, Medicare would not reimburse Aducanumab except a few patients enrolled in another clinical trials to evaluate its safety and efficacy. Biogen decided to withdraw Aducanumab from the market. Likewise, Lecanemab has not gained much acceptance in the US market. At present there is no disease modifying drug that is available to treat Alzheimer’s disease. Lloyd was the inventor and developer a new drug known as NA-831 for the treatment of Alzheimer’s disease and other neurodegenerative diseases. He was the pioneer in the new concept, known as the Neurogenesis Hypothesis. It has been discovered that in Alzheimer’s patients, the ability to regenerate new neurons in the hippocampus is impaired, causing memory loss and cognitive impairment. NA-831 facilitates neurogenesis restoring memory loss and improve cognitive functions. NA-831 which is based on mechanism of action of neurogenesis, directly related to restoring memory loss and improvement of cognitive improvement. The Phase 2A clinical data of NA-831 has shown a proof of safety and efficacy for patients with mild and moderate Alzheimer's disease. Biomed plans to conduct a Phase 2B and Phase 3 for the treatment and prevention of Alzheimer's disease. THE DEVELOPER OF OTHER NEW DRUGS Under the leadership of its Chairman and Chief Scientific Officer, Biomed's team has developed a number of new drugs for the treatment of neurological, metabolic, cardiovascular and rare diseases as given in the company’s pipeline: NA-704: for Amyotrophic Lateral Sclerosis (ALS) (Phase 2B) A-831: for Mild and Moderate Alzheimer’s Disease (Phase 2B/3) NA-901: for Major Depressive Disorder (Phase 2B/3) NA-911: for Stroke (Phase 2A) NA-921: for Rett Syndrome and Fragile X (Phase 2B/3) NA-931: for Diabetes Obesity (Phase 2B/3) NA-941: for Metabolic dysfunction-associated steatohepa For more information, please visit the website of Biomed Industries, Inc.
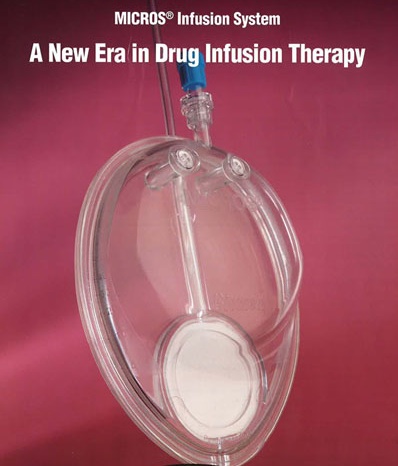
Lloyd was the inventor of a new method in therapeutic drug delivery system, having developed a novel nano-membrane as a disposable intravenous infusion device. This patented technology is known as Membrane Infusion Controlled Release Optimization System (MICROS). The MICROS is a disposable infusion pump to administer antibiotics, anti-cancer drugs and pain control medications to patients in a hospital and home care settings. The MICROS was approved for marketing in the United States by the US Food and Drug Administration.
CONTACT: To contact Lloyd Tran, please complete an online form. Thank you.. -------- © 2024. Lloyd Tran- All Rights Reserved |
|---|